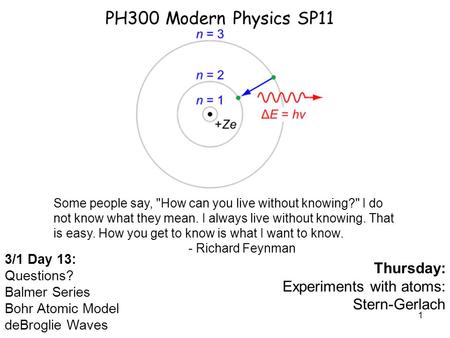
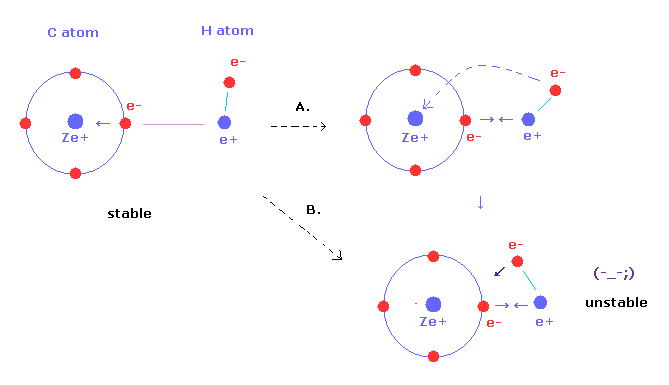
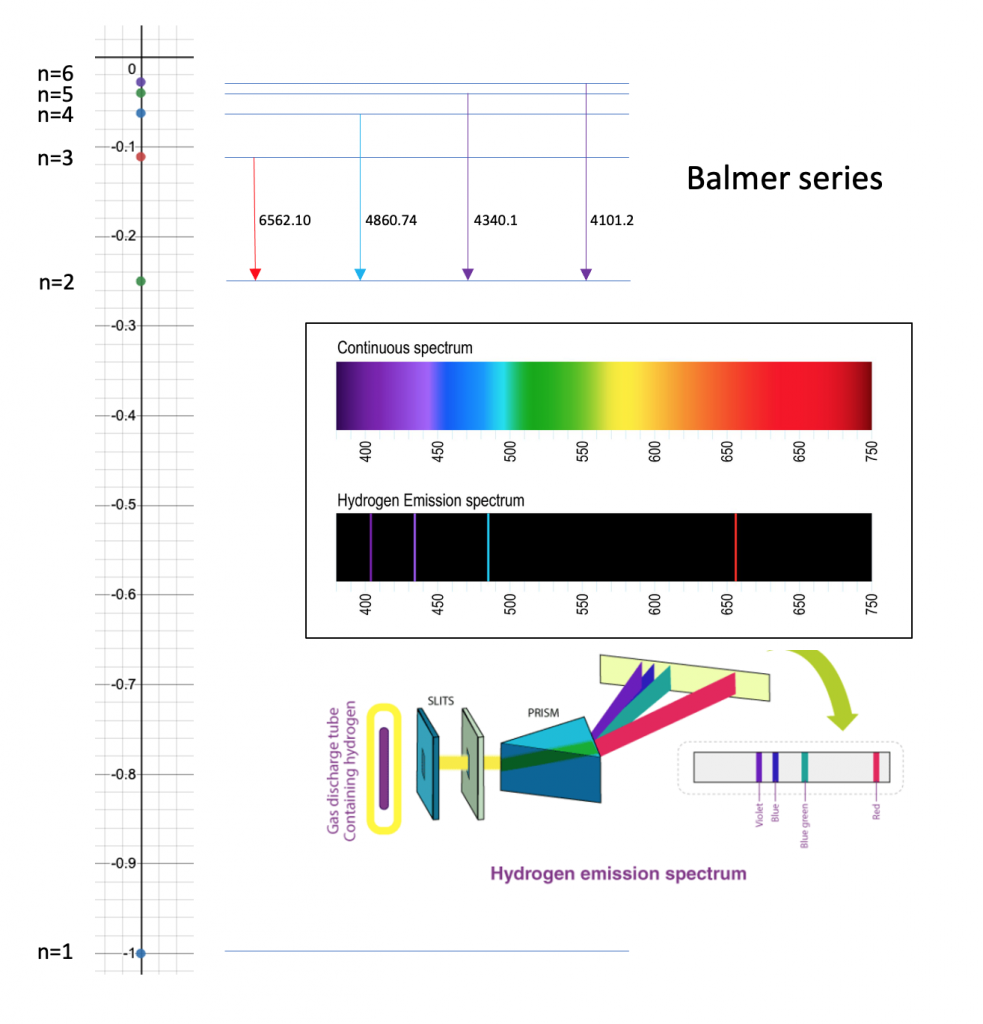
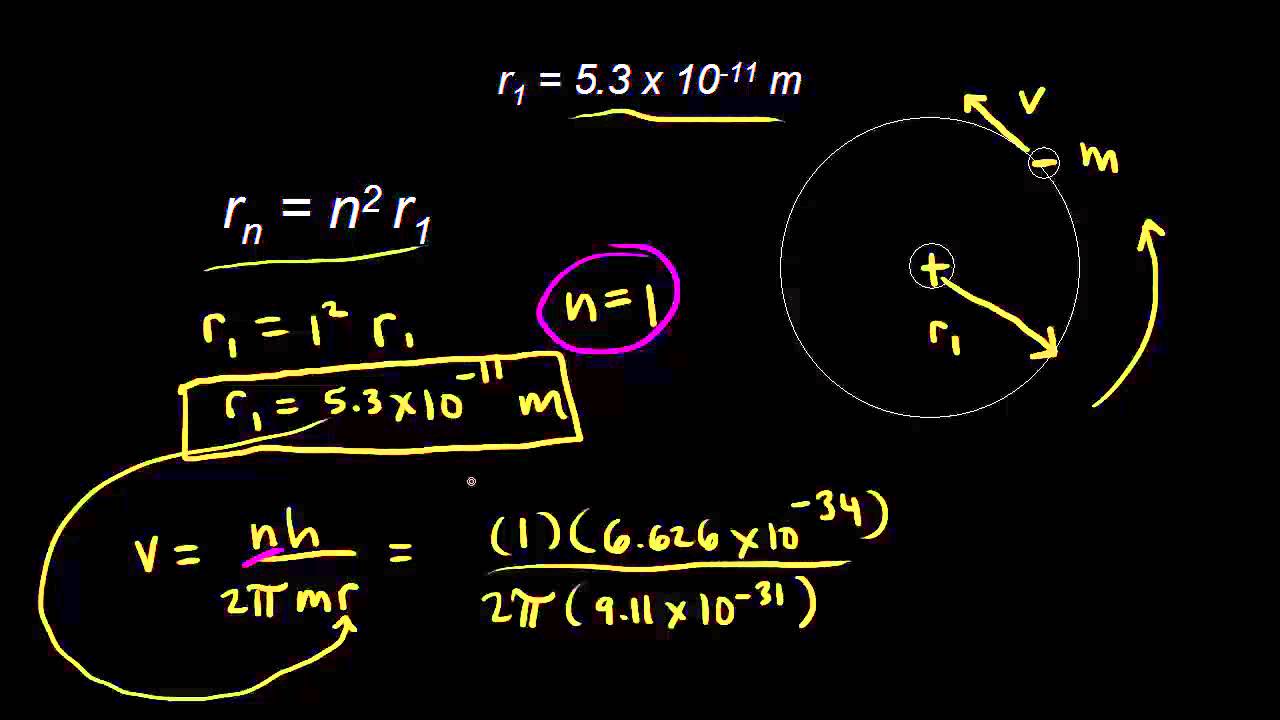Bohr Model of the Atom. Experimental Observation of Hydrogen Line Emission In 1853, Anders Angstrom of Sweden first determined that a set of discrete. - ppt download
Calculate the wavelength in Angstroms of the photon that is emitted when an electron in the Bohr's orbit, n = 2 returns to the orbit, - Sarthaks eConnect | Largest Online Education Community
According to Bohr's theory, the radius of the first orbit in a hydrogen atom is 0.528 Å. What is the radius of the fourth orbit? - Quora

The energy of an electron in the first Bohr orbit for hydrogen is - 13.6 eV. Which one of the following is a possible excited state for electron in Bohr orbit of
![The kinetic energy of an electron in the second Bohr's orbit of a hydrogen atom is: [a0 is Bohr's radius] - Sahay Sir The kinetic energy of an electron in the second Bohr's orbit of a hydrogen atom is: [a0 is Bohr's radius] - Sahay Sir](https://sahay.guru/wp-content/uploads/2020/11/17ans-1024x515.jpg)
The kinetic energy of an electron in the second Bohr's orbit of a hydrogen atom is: [a0 is Bohr's radius] - Sahay Sir
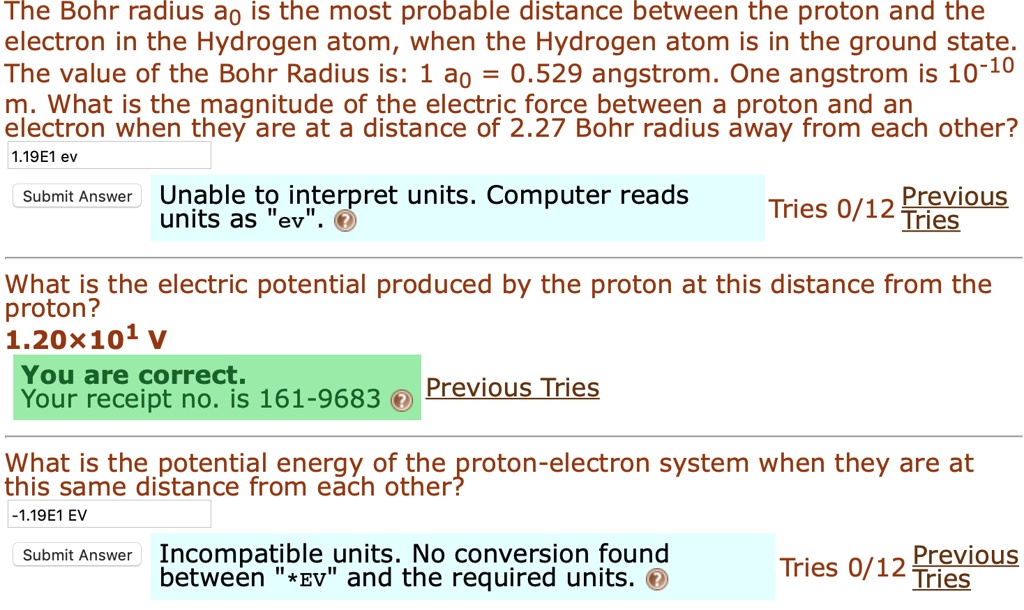
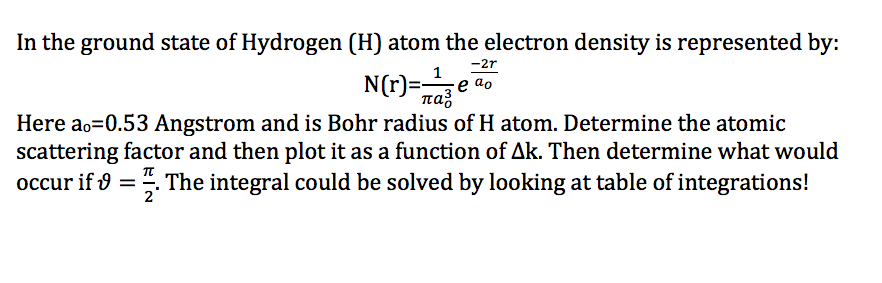
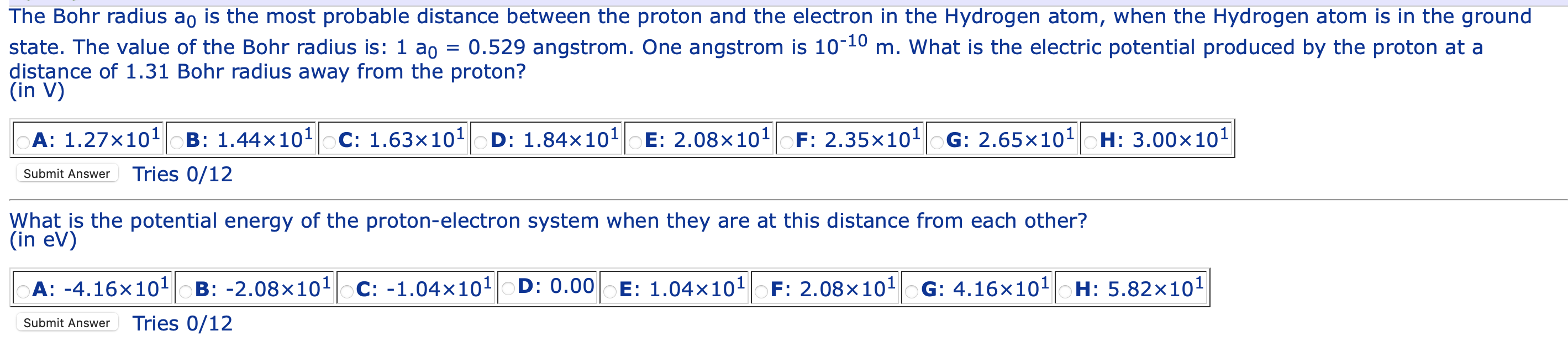
SOLVED:The Bohr radius a0 is the most probable distance between the proton and the electron in the Hydrogen atom, when the Hydrogen atom is in the ground state The value of the

6.25 | What is the radius, in angstroms, of the orbital of an electron with n = 8 in a hydrogen atom - YouTube
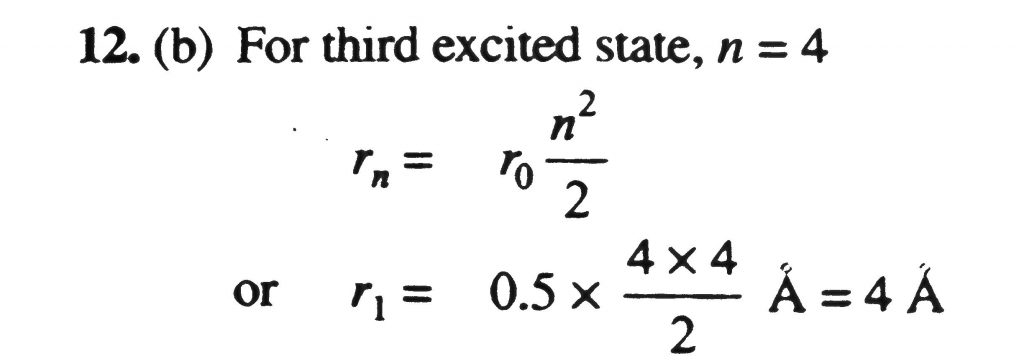
The radius of the Bohr orbit in the ground state of hydrogen atom is 0.5Å. The radius of the orbit of the electron in the third excited state of He+ will be -

3 Calculate the wavelength in angstrom of thephoton that is emitted when an electron inBohr orbit n=2 - Brainly.in